Designing 3D Vascular Networks for Tissue Engineering
Tissue engineering at clinically relevant scales requires the incorporation of perfusable channels to emulate the vasculature, providing nutrients and oxygen and removing waste products. When manufacturing techniques are developed and demonstrated at small scale the design methods are often very simple and do not scale well or have simple ways to include multiple networks, e.g. fractals and lattices. Other approaches, such as reconstructing scan data, are often difficult to work with and highly constraining. To enable the construction of large-scale tissue constructs in arbitrary volumes, we work on software to procedurally generate an arbitrary number of biomimetic networks subject to manufacturing constraints, and verify these designs in the lab.
Collaboration
We are happy to create custom designs for tissue engineering research - contact Andrew Guy with a description of the desired geometry, number of networks and relevant manufacturing constraints and Athina Markaki to establish the collaboration. We are also happy to collaborate with groups from other fields and can arrange to deliver either the libraries or a complete piece of bespoke software for mass-generating custom networks.
Examples and features
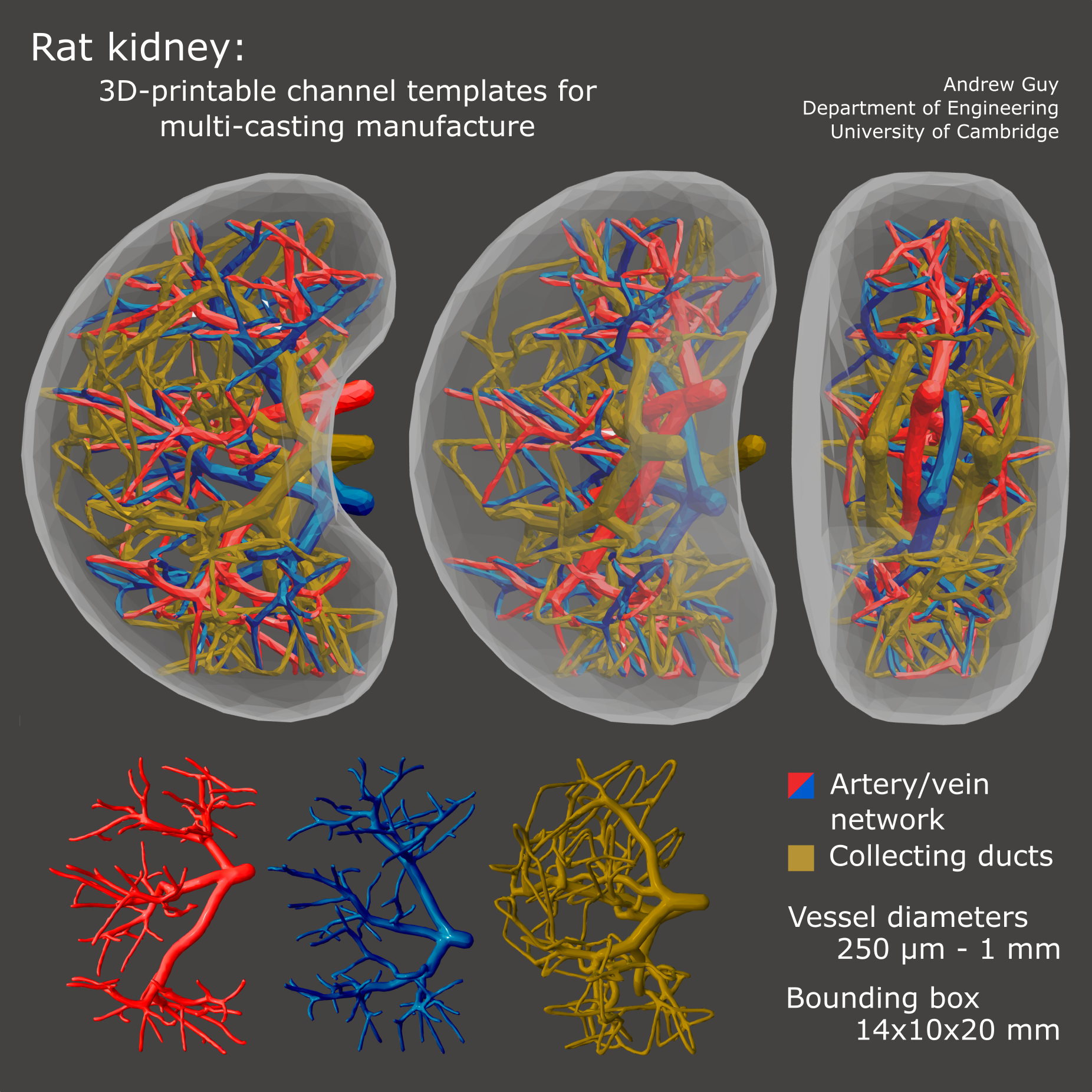 |
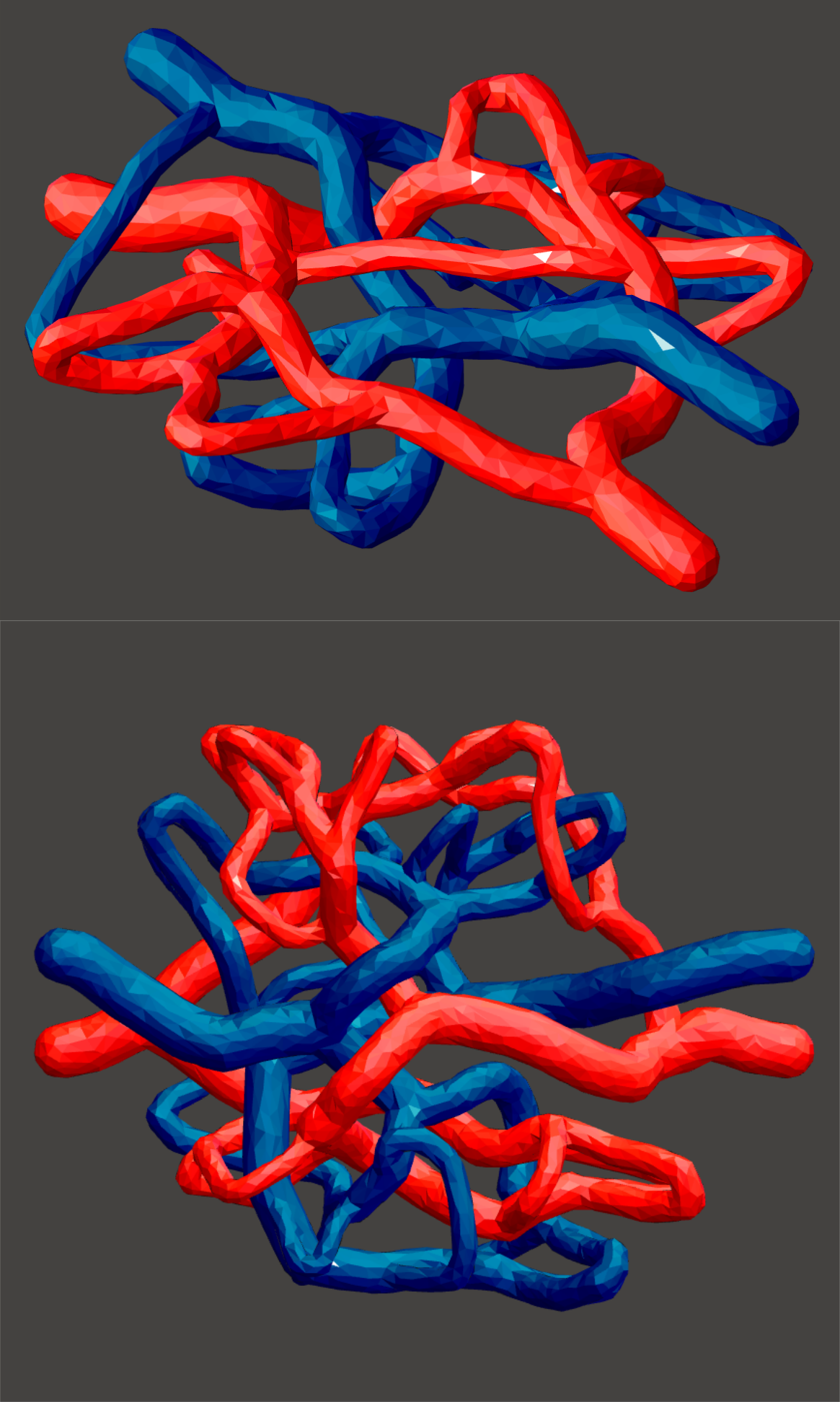
3D-printable, fluidically-distinct channel templates for vasculature and collecting ducts, procedurally generated inside a hand-modelled bounding geometry of rat kidney size and shape. Vessel diameters range from 1 mm to 250 μm, and all non-connected vessels are spaced by at least 250 μm, with spacing increasing around the larger vessels where pressure differences are higher. Note: in a multi-casting approach, single trees cannot be washed out, so there are two sets of collecting ducts forming a complete circuit. |
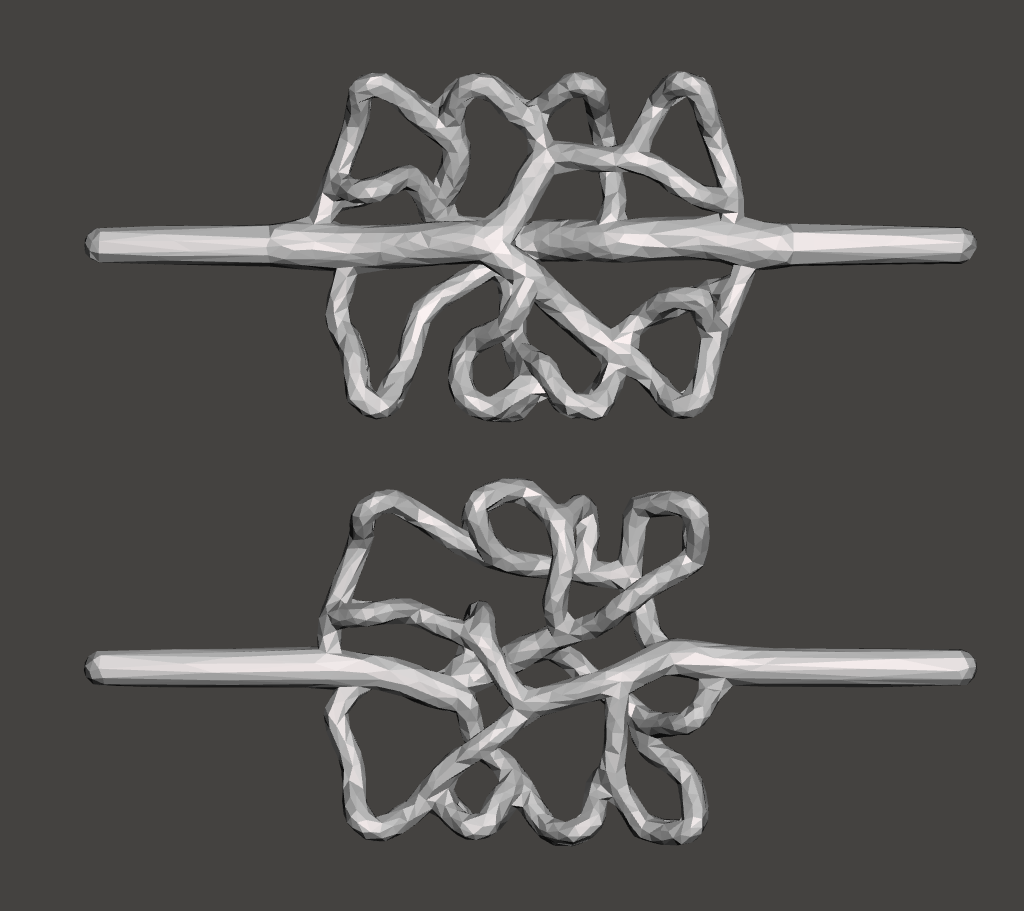
| Large numbers of candidate models may be rapidly generated in a parameter sweep, including inlet radius, bifurcation law parameters, minimum feature size and disjoint-vessel padding. These parameters may be manipulated to satisfy constraints at the expense of biomimicry. | |
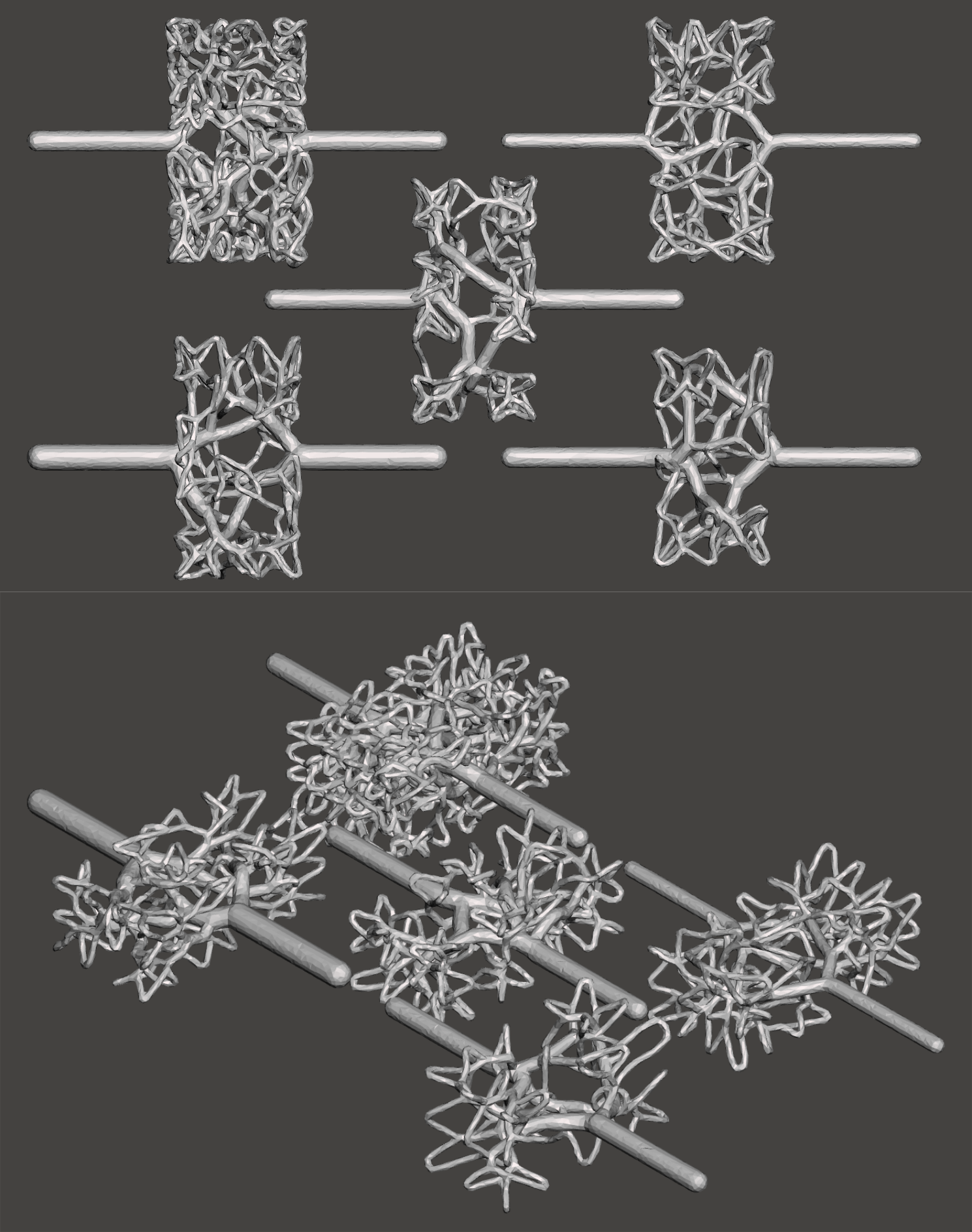
| Any number of networks may be incorporated, limited only by the amount of space and the degree to which terminal nodes removal is acceptable – near large vessels, more terminals will be removed to create space. Limits on the asymmetry at bifurcations may be specified – when high symmetry is desired the networks will double-back on themselves to supply regions of space near the inlets. | |
| A tissue engineering first approach: a focus on practical considerations such as inlet connections and manufacturing constraints. |